🌿 Find Relief with SPRAVATO® – A New Option for Treatment-Resistant Depression
If you’ve tried two or more antidepressants without the results you need, you’re not alone.
SPRAVATO® (esketamine) nasal spray is an FDA-approved treatment for adults with treatment-resistant depression or major depression with suicidal thoughts.
Why SPRAVATO® Might Be Right for You:
Works differently from traditional antidepressants
May help improve symptoms in hours or days, not weeks
Taken in a safe, comfortable setting under medical supervision
Part of a personalized treatment plan designed just for you
"When depression has resisted everything else, SPRAVATO® offers a new path forward—helping hope return, one step at a time."
-
What Is Spravato?
Spravato is the brand name for esketamine, a nasal spray medication approved in the United States for treatment-resistant depression (TRD) in adults. It can be used either alone (monotherapy) or with an oral antidepressant. spravatohcp.comspravato.comJNJ.com
It is also indicated for treating depressive symptoms in adults with major depressive disorder (MDD) accompanied by acute suicidal ideation or behavior, but only when used together with an oral antidepressant. spravatorems.comMayo ClinicJNJ.com
Esketamine acts as a non-competitive NMDA (N-methyl-D-aspartate) receptor antagonist, distinguishing it from traditional antidepressants by targeting glutamate pathways in the brain. WikipediaJNJ.com
-
Effectiveness & How It Works
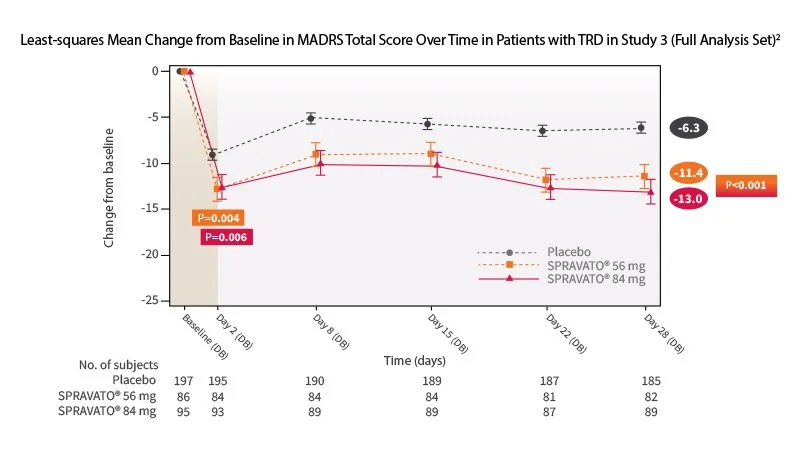
Spravato offers rapid relief, with some patients experiencing improvement within 24 hours, and more pronounced effects by 28 days. Verywell HealthJNJ.comReutersNew York Post
Clinical trial data show that, when used alone, 22.5% of patients reached remission at four weeks, compared to 7.6% with placebo. Verywell HealthJNJ.comReuters
In Europe, evidence indicates that used alongside an SSRI or SNRI, Spravato® helps reduce relapse rates—from 45% with placebo down to 27% with Spravato®.
-
Administration & Dosing Protocol
Initial Phase (Weeks 1–4):
1–2 sprays per nostril on the first day.
Then, 1–3 sprays per nostril twice weekly for the next 4 weeks.
Continuation Phase (Weeks 5–8):
Once-weekly dosing if improvement is observed.
Maintenance Phase (after Week 8):
Continued use once a week or every two weeks, depending on the patient's needs and physician guidance. European Medicines Agency (EMA)Mayo Clinic
Blood pressure is routinely monitored before and after dosing, and treatment is not recommended for patients with certain cardiovascular or cerebrovascular conditions. European Medicines Agency (EMA)Mayo Clinic
-
Side Effects & Precautions
Common adverse effects (affecting up to ~30% of users): dizziness, nausea, dissociation, headache, sleepiness, vertigo, taste changes, numbness, vomiting. European Medicines Agency (EMA)
Other potential concerns include sedation, perceptual alterations, elevated blood pressure, and—rarely—bladder issues like ulcerative or interstitial cystitis. WikipediaMayo ClinicEuropean Medicines Agency (EMA)
Patients should not drive or operate machinery until the next day or until they are fully alert. Mayo Clinic
-
Regulatory & Safety Measures
Because of risks like sedation, dissociation, respiratory depression, and abuse potential, Spravato® is only available under a restricted program called REMS (Risk Evaluation and Mitigation Strategy).
Patients must receive treatment at certified healthcare settings and are monitored for a minimum of 2 hours post-dose